Trials and trifluoromethylations





23rd September 2020
Establishing new in-house methods to increase the range of available catalogue compounds is one of our key objectives in the Apollo Labs. We have recently worked on the selective mono-trifluoromethylation of highly volatile 1,2-diketones. Similar compounds have been made via trifluoromethylations using Ruppert’s reagent in neat conditions.
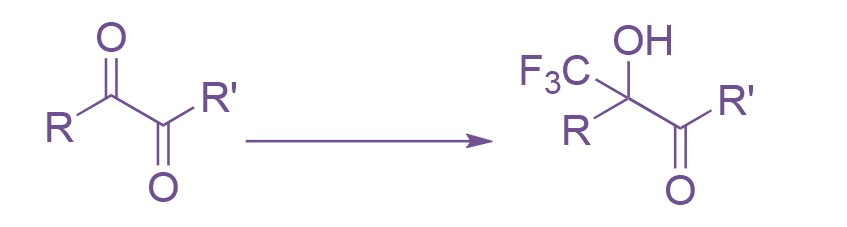
Scheme 1: The initial reaction
Focusing on 2,3-butadion we trialled literature conditions and observed a VERY lively exothermic reaction resulting in a nice black tar. As always never deterred we went on and trialled several reaction conditions (temperature, equivalents, reagents, order of addition, solvent); and in the end we developed a two step process which is safe (neglectable exotherm and moderate gas evolution) and gives reproducible yields for both steps. We have scaled the process to 150g of PC50170 and are now expanding the range to other diketones.
The TMS ether PC50974 can be purified and both items are now available from stock.
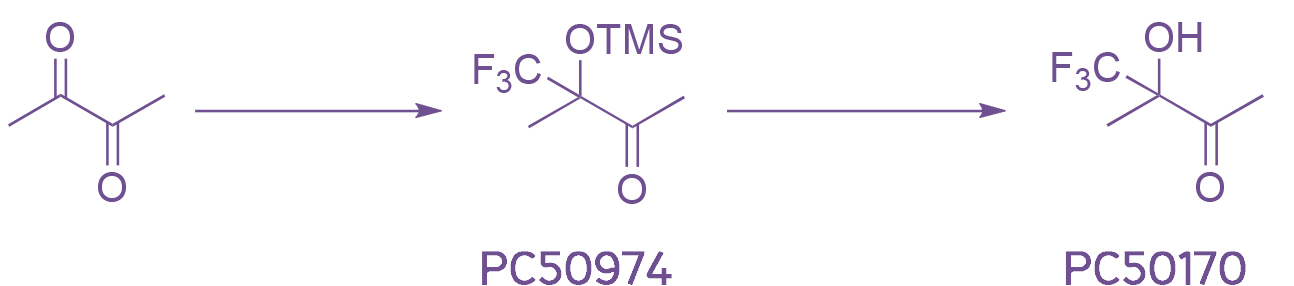
Scheme 2: The final two step process
Apollo Scientific are a leading manufacturer and supplier of high quality research chemicals, supporting discovery across the global market.
We serve clients across a wide range of industries, with a diverse product portfolio and a high success rate on unproven chemistry, allowing you to source novel building blocks, or outsource synthesis of raw materials and key intermediates.
As one of the UK’s most established chemical distributors, we have a global reach.
With more than 25,000 products in stock and over 100,000 products listed on our website, look no further to buy research chemicals, organic building blocks, life sciences reagents, high purity inorganics, deuterated solvents, and spectroscopy consumables.
To speak to a member of our expert team about research chemical supply, manufacture or distribution email sales@apolloscientific.co.uk or call +44 (0)161 406 0505